Lipid NanoParticle (LNP) Formulation
Featured Products
Information
Delivering the capabilities pharma needs to support its drug strategies, CordenPharma offers advanced LNP formulation technologies and forward-integrated sterile injectable manufacturing services to create a proven commercialization pathway.
We support requirements for Pre-filled Syringes (PFS), Vials, Ampoules and Lyophilized Vials, with a wide range of filling volumes and secure supply with highly compatible excipients and reliable synthesis.
Our cGMP processing and manufacturing strategies are based on ethanol injection, including in‐line T‐junction mixing (used to mix an organic and aqueous phase in a controlled manner) as well as a microfluidic mixing method.
These methods work using the same principle for the formation of LNPs:
- Formed by a quick increase in polarity of the environment induced by rapid mixing of the two miscible phases. This rapid mixing induces supersaturation of lipid molecules.
- Self‐assemble into the desired structure without the need for size‐reduction methods.
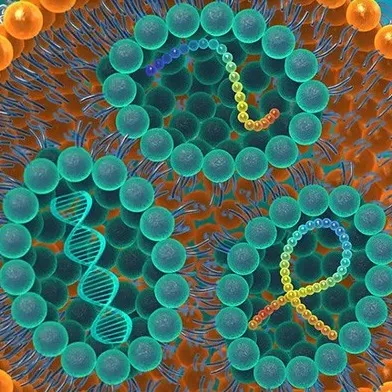
Creating Better Lipid NanoParticles for Vaccine Innovation
Lipid NanoParticles are liposome-like structures composed primarily of cationic lipids, along with other lipid ingredients. These typically include neutral phospholipid molecules especially geared towards encapsulating a broad variety of genetic payloads:
- DNA-RNA based nucleic acids
- siRNA, mRNA and saRNA
- CRISPR / Cas9 genome‐editing
Proven LNP Formulation & Commercialization Pathway
To enable the capabilities pharma needs to support today’s mRNA and any other x-RNA drug strategies, CordenPharma continues to invest in advanced LNP formulation technologies and sterile injectable manufacturing services:
- Expanded R&D laboratory LNP Formulation & Analytical Characterization capabilities
- Flexible & scalable cGMP LNP Formulation for clinical to commercial supply
- Fully approved Sterile Injectable manufacturing site able to produce Drug Products from clinical to commercial stage
Main Categories
Custom ManufacturingExcipientsFinished Dosage FormsAPIs (Active Pharmaceutical Ingredients)
All Categories
Drug Delivery Devices & SystemsExcipientsFilling/ Fill Finish & packagingLipid Modifying Agents
Distribution areas
North America (USA, Canada)East Asia (e.g. China, Japan, Korea)Europe - EU countriesSouth Asia (e.g. India, Pakistan, Sri Lanka)
Supplied from
Italy
