Translational Pharmaceutics
Products
Information
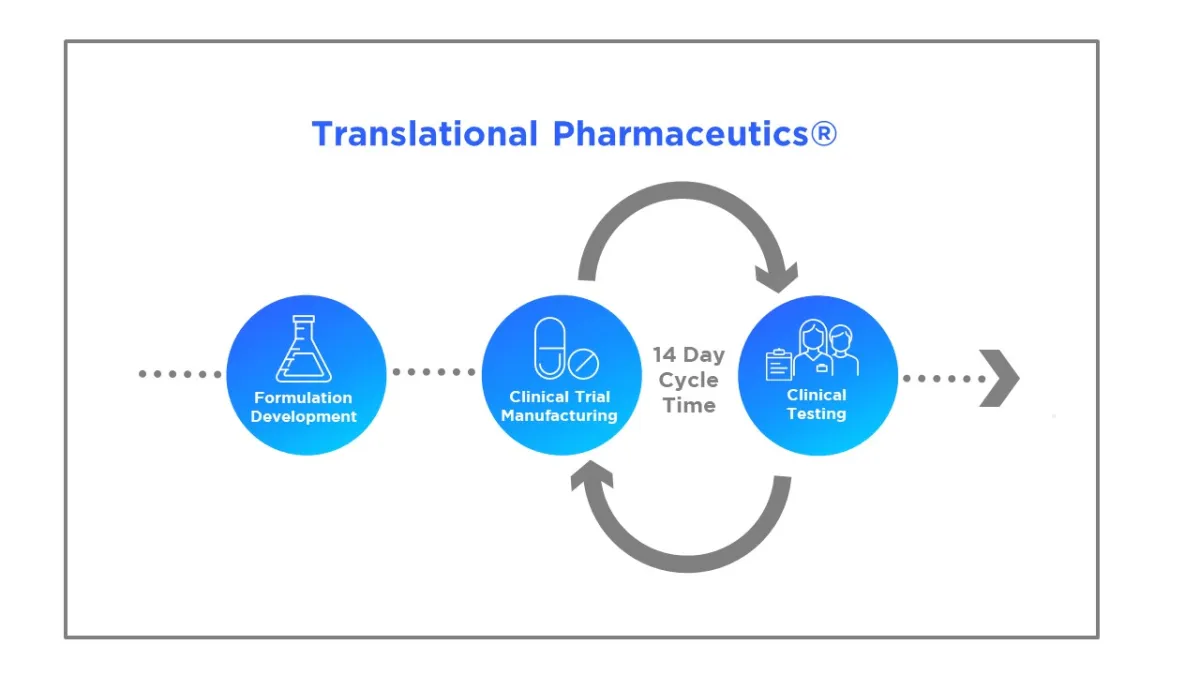
Translational Pharmaceutics(R), accelerates development by integrating formulation development, real-time manufacturing and clinical testing. This integrated approach is proven to reduce timelines by more than 12 months and lower R&D costs by more than $100 million. Over the past decade it has been used widely by pharmaceutical and biotech companies on >400 programs to advance molecules across the full development cycle from first-in-human trials to market (FIH) Key applications within development process include:Transitioning molecules from first-in-human (FIH) to proof-of-concept (POC) and the development and optimization of clinical formulations. To access the latest Tufts CSDD white paper analyzing the benefits of Translational Pharmaceutics visit: https://www.quotientsciences.com/solutions/translational-pharmaceutics/tufts-report/
Main Categories
Custom ManufacturingContract Services - Analytical & Lab ServicesPharmaceutical Machinery & Technology
All Categories
Custom ManufacturingCustom Manufacturing of Dosage Form DrugsDrug Formulation (general category)Contract Services - Analytical & Lab ServicesFormulation developmentPharmaceutical Machinery & Technology
Supplied From
United KingdomUnited States
