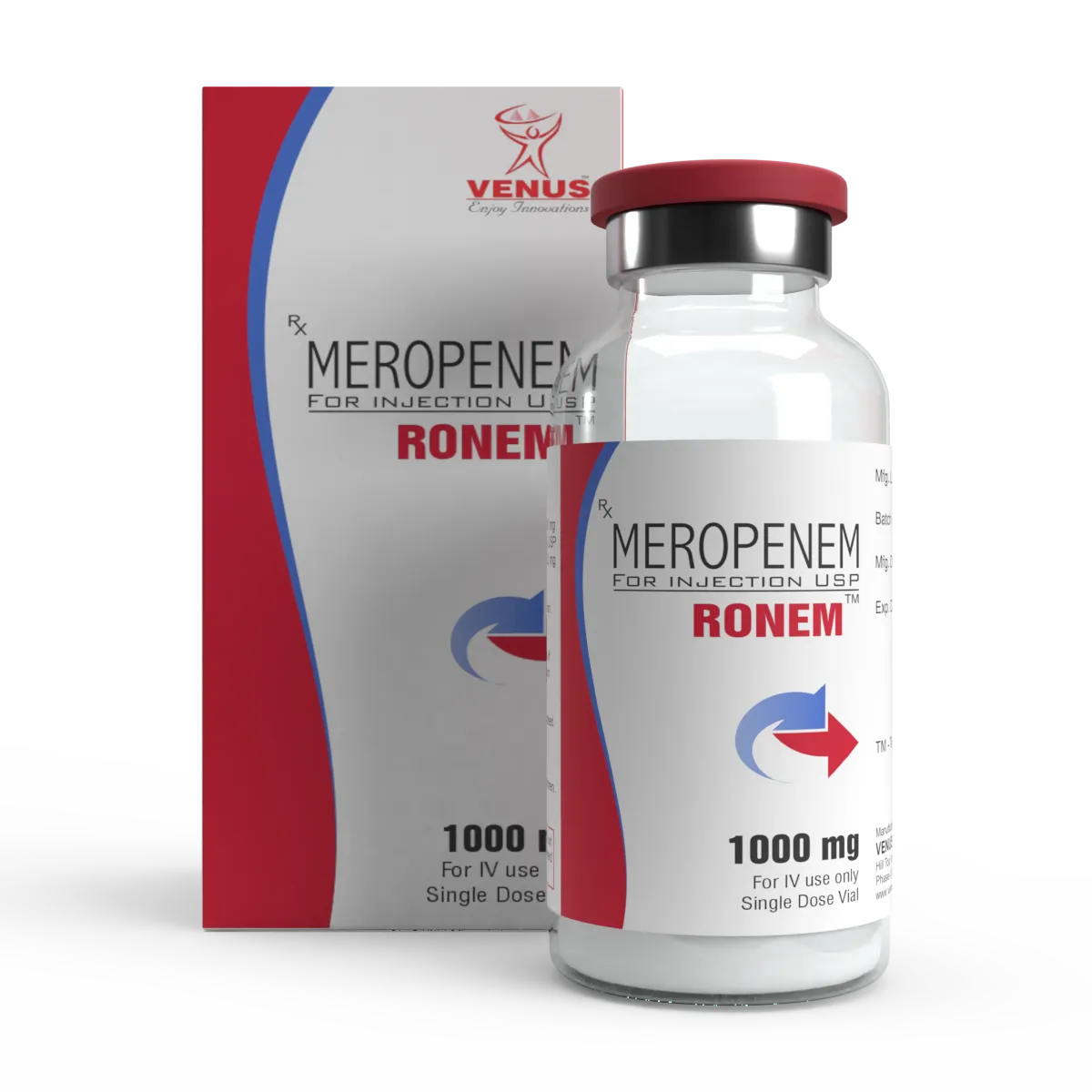
ANTIBIOTIC INJECTABLES Meropenem(FOR CRITICAL CARE)
Products

Information
Meropenem injectable formulations, developed under stringent International regulatory standards. Our flexible manufacturing capabilities allow us to cater to partner-specific requirements across global markets — whether for direct product supply or as a trusted third-party / contract manufacturing partner. Product Highlights: API Strengths Available: 500 mg, 1 g & 2 g Dosage Form: Sterile powder for injection (vial) Packing Options: Glass vials with flip-off seals and tear-off aluminum caps, available in single vial mono, combi packs(WFI) or 10's vials pack Customization: Product formulation, packaging, labeling, and documentation tailored to regulatory and commercial requirements of partner markets.Marketing support through literatures and Trainings for sales teams, CME presentations etc. could also be arranged. Shelf Life: 24 months (standard); stability studies available for custom specifications.
All Categories
Injectables
Main Categories
Finished Dosage Forms (Products)
Distribution Areas
OceaniaNorth America (USA, Canada)AfricaMiddle East Region (e.g. UAE)Central America (e.g. Mexico)Europe - EU countriesEurope - non EU (e.g. UK, Russia, ex-CIS countries)South America (e.g. Brazil, Colombia)South East Asia (e.g. Thailand, Philippines, Singapore)
Supplied From
GermanyIndia
