
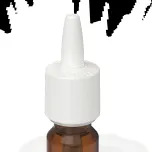
Gemini(TM) BE Nasal Pump
Products

Information
When healthcare companies seek alternative device suppliers, Bioequivalence (BE) testing provides a measure of assurance that the product formulation will perform the same as a reference medicine. With respiratory diseases and infections on the rise, this state-of-the-art testing allows our customers to overcome regulatory and technical barriers that previously slowed down product commercialization. With Silgan Dispensing's BE Program, we can develop expedited nasal devices to fit your needs as a replacement or secondary supply chain option. Our quick time-to-market nasal pump has over 40 possible spray configurations, providing multiple possibilities to match the reference drug/device combination. In addition, our BE program is aligned with U.S. FDA and EU methods - providing customers the confidence they require from a healthcare packaging supplier.
All Categories
Primary Packaging (Packaging Materials and Equipment) (Products)Medical Devices and Medical Packaging
Main Categories
Packaging Materials and Equipment (Products)
