Kemwell Biopharma
Products


Information
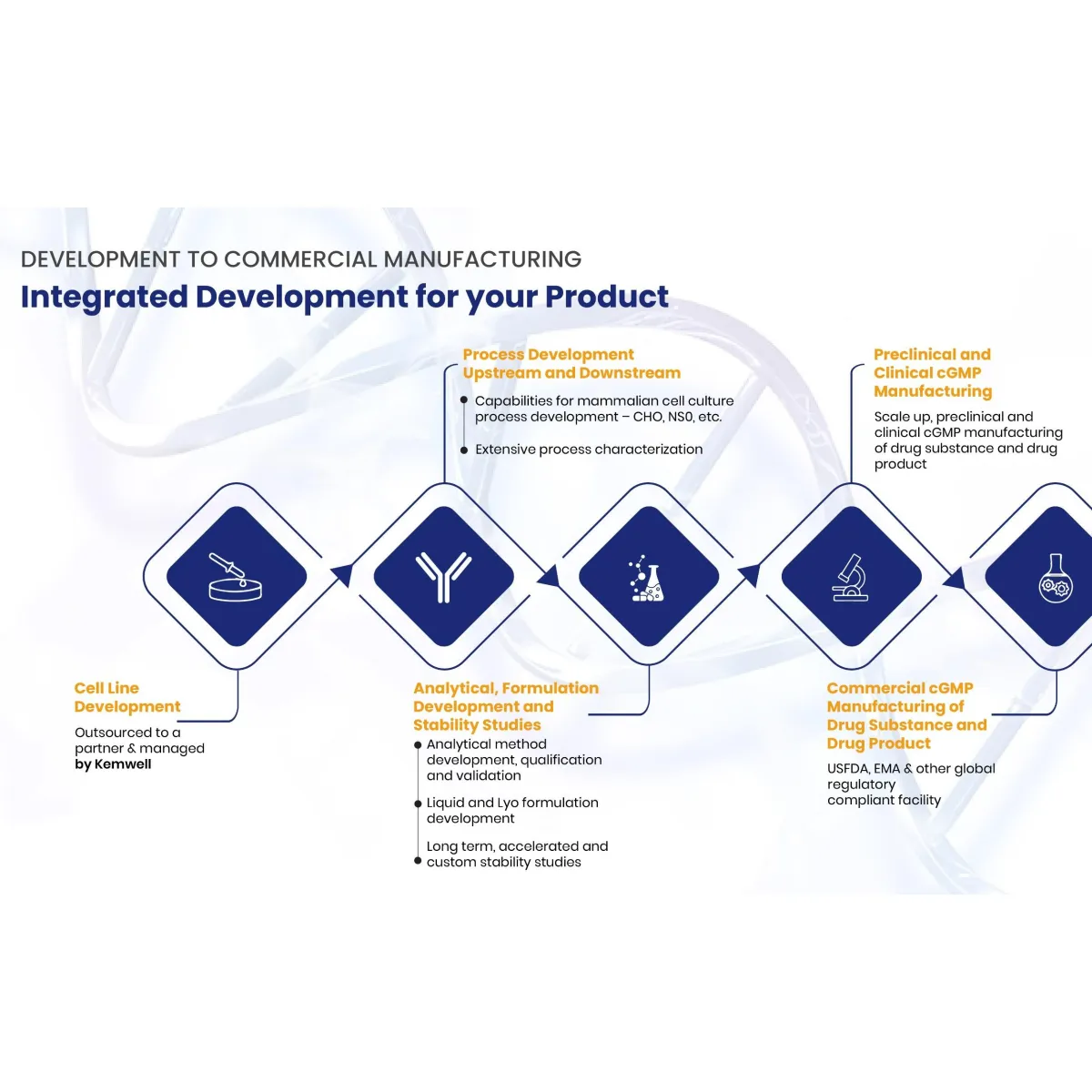
SERVICES Kemwell provides integrated development and manufacturing services for companies that require one-stop solution for mammalian cell-culture based protein therapeutics The team is experienced to undertake end-to-end activities right from cell line development till cGMP clinical and commercial manufacturing. Kemwell also undertakes standalone projects such as process optimisation, analytical method development or validation, formulation development and optimization, stability studies, resin reuse study, tech transfer and cGMP manufacturing, process characterization, etc.
All Categories
BiosimilarsAntibodiesAnalytical DevelopmentBio Analytical ServicesChemistry, Manufacturing and Controls (CMC)Antibody Drug Conjugates (ADCs)
Main Categories
Biopharmaceutical ProductsContract Development and Manufacturing Organisations (CDMOs) and Contract Manufacturing Organisations (CMOs) (Services)Innovative APIsContract Research Organisations (CROs) (Services)
Distribution Areas
North America (USA, Canada)East Asia (e.g. China, Japan, Korea)Europe - EU countriesEurope - non EU (e.g. UK, Russia, ex-CIS countries)South Asia (e.g. India, Pakistan, Sri Lanka)
