System Qualification
Products
Information
Comprehensive system check during FAT and after commissioning.
Reliable quality right up to the final test
After installation and commissioning at the customer's premises, further extensive tests are carried out, which are documented in the IQ/OQ qualifications (Installation Qualification & Operation Qualification). These are mandatory in the pharmaceutical and medical industries and ensure that all processes comply with GMP guidelines.
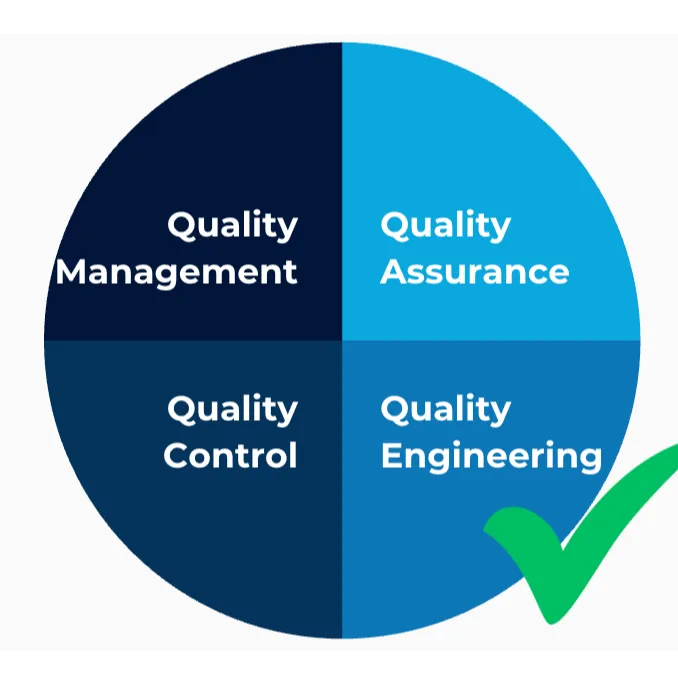
Our quality department is divided into four specialized areas:
- QA (Quality Assurance): Recording and processing of customer complaints using an 8D report
- QE (Quality Engineering): Development of new measurement methods in cooperation with our ISO 17025-accredited laboratory
- QC (Quality Control): Testing and implementation of new measurement methods in daily operations
- QM (Quality Management): Further development of systems, training, supplier qualifications & management reviews
Quality that you can also feel in the support
If challenges arise when using our software or hardware, our competent support team is ready to help. Together with the quality department, we will quickly and efficiently find solutions to ensure the smooth operation of your systems.
- Our experts are highly trained and available to you at all times
- Problems are analyzed directly and solved sustainably
- Your success and satisfaction are our top priority
Put your trust in our quality management - for reliable systems, optimized processes and long-term customer satisfaction.
