Document Management System (DMS) and Training Management System(TMS)
Products and Business Services
Information
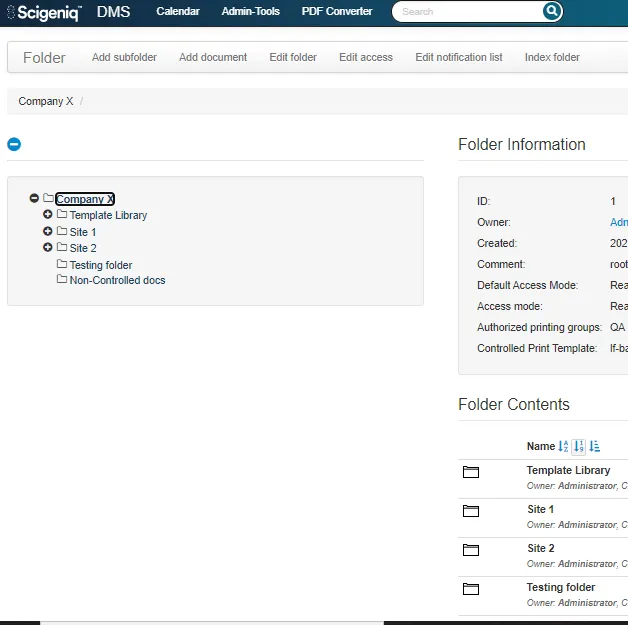
Scigeniq DMS is a comprehensive document management system designed for life sciences companies. It provides an organized, centralized repository for all types of documents, from SOPs to manufacturing drawings.
With Scigeniq DMS, documents are protected, tracked, and managed to ensure compliance with regulatory requirements. The system includes complete workflows for creating, reviewing, and approving documents within a CSV-compliant, validated environment.
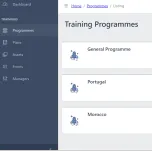
Scigeniq Training Management System (TMS) is a complete training management system that enables pharmaceutical companies to plan, track, and report on required employee training.
Scigeniq TMS ensures compliance with rigorous industry regulations by centralizing and automating a company’s training program. A complete training record is built for each employee, documenting the training they require based on their role, their completed training, and any gaps between the two.
Scigeniq TMS automates the entire training process. For example, when new SOPs are created or new processes are defined, a training requirement for every affected employee is created. The training asset – a presentation, a video, or a document, for example – is automatically sent to each person who requires the training with a date for completion. After the employee has completed the required training, a record of their having received, read, and understood the SOP or process is created.
Supplied From
BrazilJordanSaudi ArabiaUnited Arab Emirates
