Regulatory Management System (RMS)
Products and Business Services

Information
Scigeniq RMS is an all-inclusive, automated system for planning, tracking, coordinating, and documenting regulatory submissions for life sciences companies.
Scigeniq RMS provides an extensive set of capabilities to manage all aspects of the regulatory submission process, from initial planning to submission and archiving. And with Scigeniq RMS, submission activities are organized around best practices and address all relevant regulatory requirements.
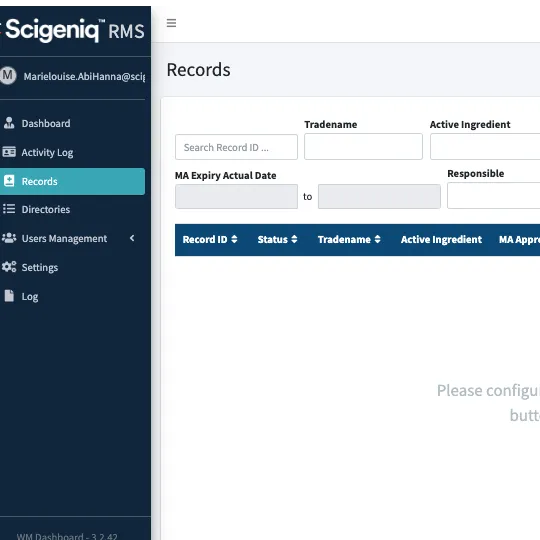
International Registration Tracking
Captures and records all product registrations for multiple jurisdictions and stores all documents, correspondence and dossiers, and marketing authorizations.
Plan & Track Tool
Includes the ability to easily export to a spreadsheet for tracking of approvals versus plans, deliverables, pending actions, and setting plans for submission activities months ahead.
Regulatory Archiving System
Stores the complete set registration dossiers, creating a complete record of the registered product, and allowing easy access to all users for compliance checks.
Distribution Areas
Middle East Region (e.g. UAE)Central America (e.g. Mexico)Europe - EU countries
Supplied From
JordanUnited Arab Emirates
